
With three degradation profiles (Fast, Medium and Slow)

The ARCHIMEDES stent is a Biodegradable Biliary and Pancreatic stent intended to be used to drain obstructed biliary or pancreatic ducts. The patented Dual Drainage Design Helical of the stent allows for bile to flow on the outer surface of the device while supporting the opening of the lumen.
Developed and designed to be placed endoscopically, percutaneously, or surgically, either open or laparscopic surgery, the ARCHIMEDES provides a flexible and innovative solution that can help reduce complications and the costs associated with them by removing the need for unnecessary additional clinical interventions.

* The different degradation profiles are designed for obstructed biliary or pancreatic ducts with various underlying diseases.
** Minimal Strength Retention is defined by the presence of at least 10% of an initial strength parameter. The Stent remains intact with no breaks, tested in a simulated degradation model.
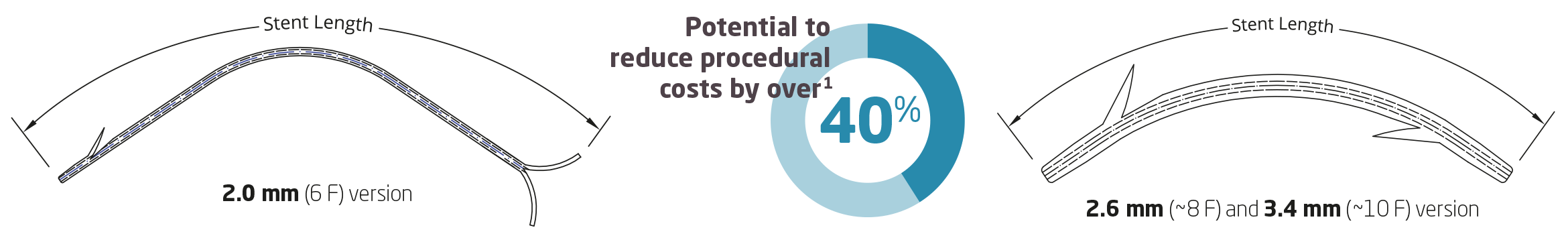
1 2019 Frost & Sullivan Independent Market Research Report.
2 The different degradation profiles are designed for obstructed biliary or pancreatic ducts with various underlying diseases.
3 Minimal Strength Retention is defined by the presence of at least 10% of an initial strength parameter.
The Stent remains intact with no breaks, tested in a simulated degradation model.
The product official name is ARCHIMEDES BPS Pancreaticobiliary Stent.
Indication: Pancreatic Stent For PEP Prophylaxis. Data from Dr. Andreas Maieron report at https://www.sciencedirect.com/science/article/pii/S1590865822008593
- DOWNLOAD ARCHIMEDES Clinical Evolution - Endoscopic
- DOWNLOAD ARCHIMEDES Clinical Evolution - Surgical
For further inquiries regarding
Manufactured by amg International GmbH, Boschstraße 16, 21423 Winsen, Germany